Sterilization as a Medical Device QA Blind Spot: How to Preserve Traceability Before, During, and After
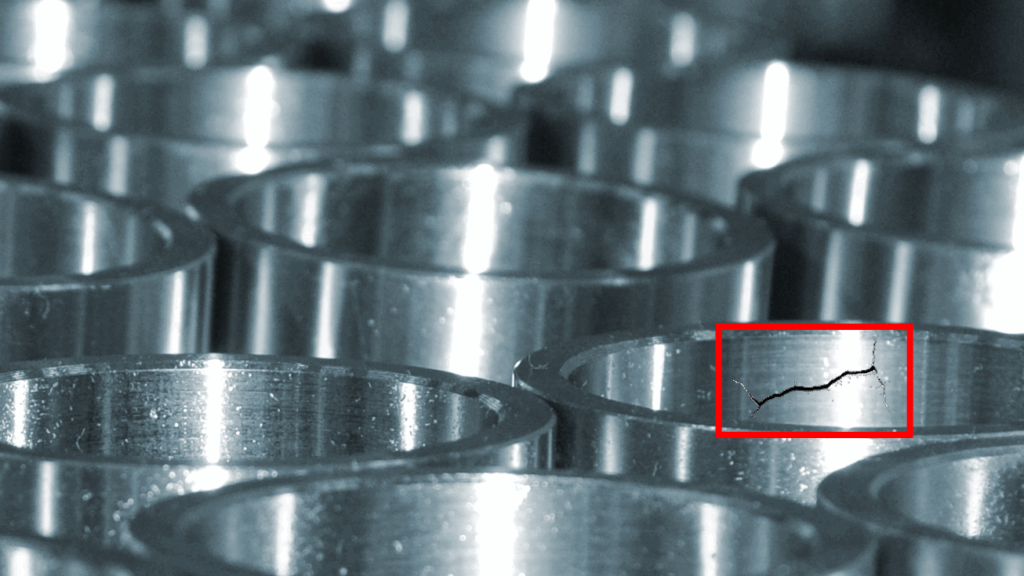
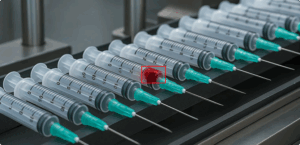
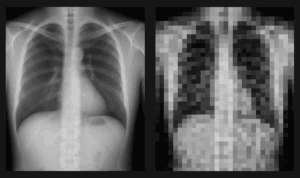
QA visibility is critical at every stage of medical device manufacturing. Sterilization is no exception, yet it’s often the point where visibility is lost. And when visibility is lost, risk creeps in. Once a device is sealed for sterilization, it can disappear from inspection until the cycle ends. This blind