Akridata Named a Vendor to Watch in the IDC MarketScape for Worldwide Data Labeling Software Learn More
Computer Vision in Medical Devices
Ensure Manufacturing Quality with AI in Medical Device Manufacturing

In 2023, there were 975 medical device recalls in the United States, impacting 283 million units and causing severe financial and reputational loss.
SOLUTION
Reliable AI-Powered Device Defect Detection for Medical Manufacturing
Goal
Improve the precision and reliability of AI in medical device manufacturing to prevent defect escapes and ensure quality production.
Problem
Manual inspections are inconsistent and often unreliable. Even classical rule-based vision systems struggle with process variability. This makes the need for robust AI-driven medical device inspections more critical than ever.
Solution
Akridata’s Vision Command uses advanced deep learning to build and deploy reliable AI inspection models tailored for medical manufacturing. These models integrate seamlessly with existing hardware and ensure early, accurate defect detection.
See how Akridata can help.
AI-Based Integrated Device Inspection System for Medical Manufacturing
Image Data Collection

AI-Powered Image Data Collection for Medical Devices
Advanced Device Inspection
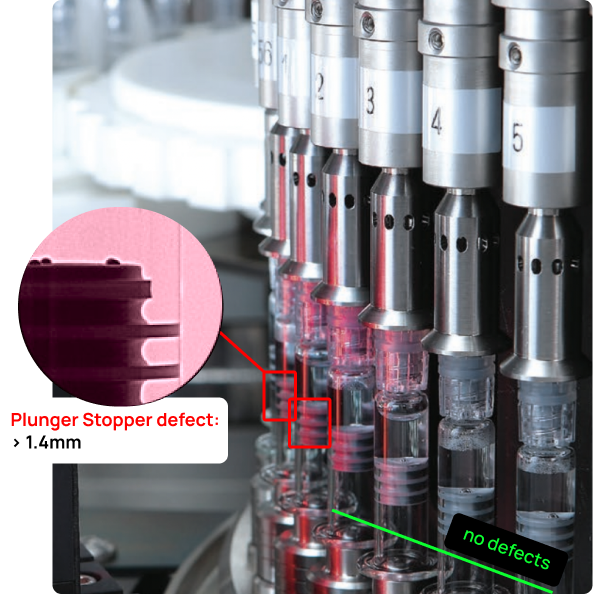
Deep Learning for Advanced Medical Device Inspection
Defect Categorization and Decision
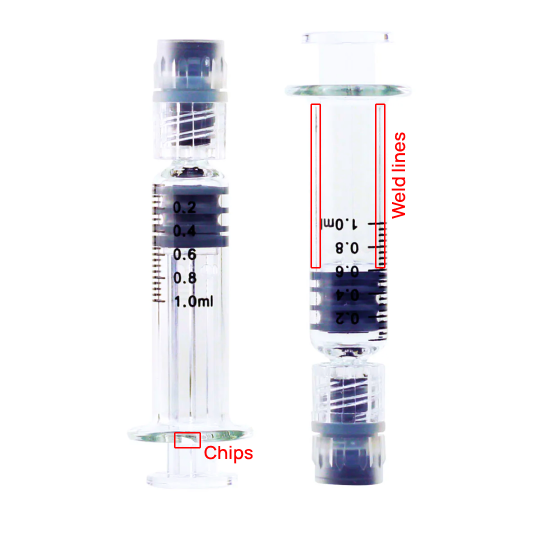
Defect Categorization & Root Cause Identification using AI
Continuous Deployment and Monitoring

AI-Based Continuous Monitoring Across Production Lines
Need More on AI in Medical Device Manufacturing?
Akridata Visual Data Platform for Medical Device AI
Don’t have an in-house Data Science team for device defect detection?
Akridata provides ready-to-use medical device models that have been rigorously tested and refined using millions of device images, ensuring accurate defect detection.
Medical Device Manufacturer Improved Accuracy by 40% Using AI in Medical Device Manufacturing
A medical device client sought to improve accuracy and efficiency in its computer vision-based inspection lines. The company's existing system fell short despite repeated model tuning. Real-world production conditions and a rigid, speed-focused approach caused incorrect part flagging, posing the challenge of maintaining speed without sacrificing accuracy or risking defective product shipments.
By leveraging Akridata Vision Command and implementing AI in medical device manufacturing, the company achieved a 40% decrease in false positives and a 30% drop in false negatives. This significantly enhanced production line efficiency, reduced waste, and safeguarded their brand reputation.