In the automotive industry, precision and quality define trust. Even the smallest surface imperfection, whether a scratch, dent, or coating inconsistency can lead to costly rework, warranty claims, and customer dissatisfaction. Traditional visual inspections, though long relied upon, struggle to keep pace with the increasing complexity, speed, and precision demands of modern automotive production lines. […]
Surface defects are one of the most persistent challenges in steel and metal manufacturing. From scratches and dents to inclusions and cracks, even the smallest anomaly can compromise product quality, increase rework costs, and damage customer trust. Traditional inspection methods – whether human visual checks or legacy machine vision – often fall short in meeting […]
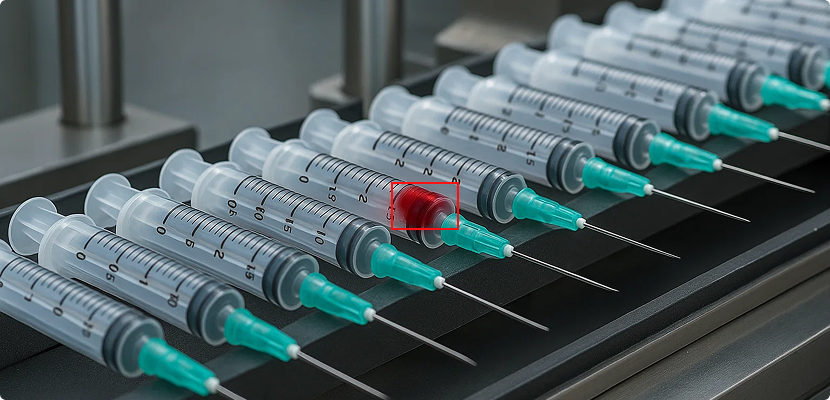
QA visibility is critical at every stage of medical device manufacturing. Sterilization is no exception, yet it’s often the point where visibility is lost. And when visibility is lost, risk creeps in. Once a device is sealed for sterilization, it can disappear from inspection until the cycle ends. This blind spot, whether in an in-house […]
When a medical device batch is rejected, the cost is staggering. A single rejection can wipe out $600K-$2M in logistics, rework, and re-sterilization costs. But the real damage goes far beyond mere dollars. Quality misses can cause strained Original Equipment Manufacturers (OEMs) and Contract Manufacturers (CMs) relationships, delay launches, and even FDA scrutiny that can […]
Manufacturers of medical devices and critical components consistently struggle with quality control. An over-reliance on manual inspection — and the inherent human error that comes along with it — regularly leads to both financial and environmental waste, as well as potential harm to consumers and to the manufacturer’s brand reputation. Today, however, automated inspection systems […]
The FDA classifies medical devices based on risk to patients, with Class I being the lowest and Class III the highest. Class II and Class III devices — such as infusion pumps, orthopedic implants, or cardiovascular catheters — carry greater patient risk, requiring more stringent design, testing, and postmarket controls. These higher classifications trigger more […]
Manufacturing has become faster, more precise, and increasingly automated – but that hasn’t made it immune to defects. In fact, the complexity of today’s production environments has introduced a new challenge: the tiniest deviation in process, machine behavior, or raw material consistency can snowball into full-blown quality failures. And by the time those failures are […]
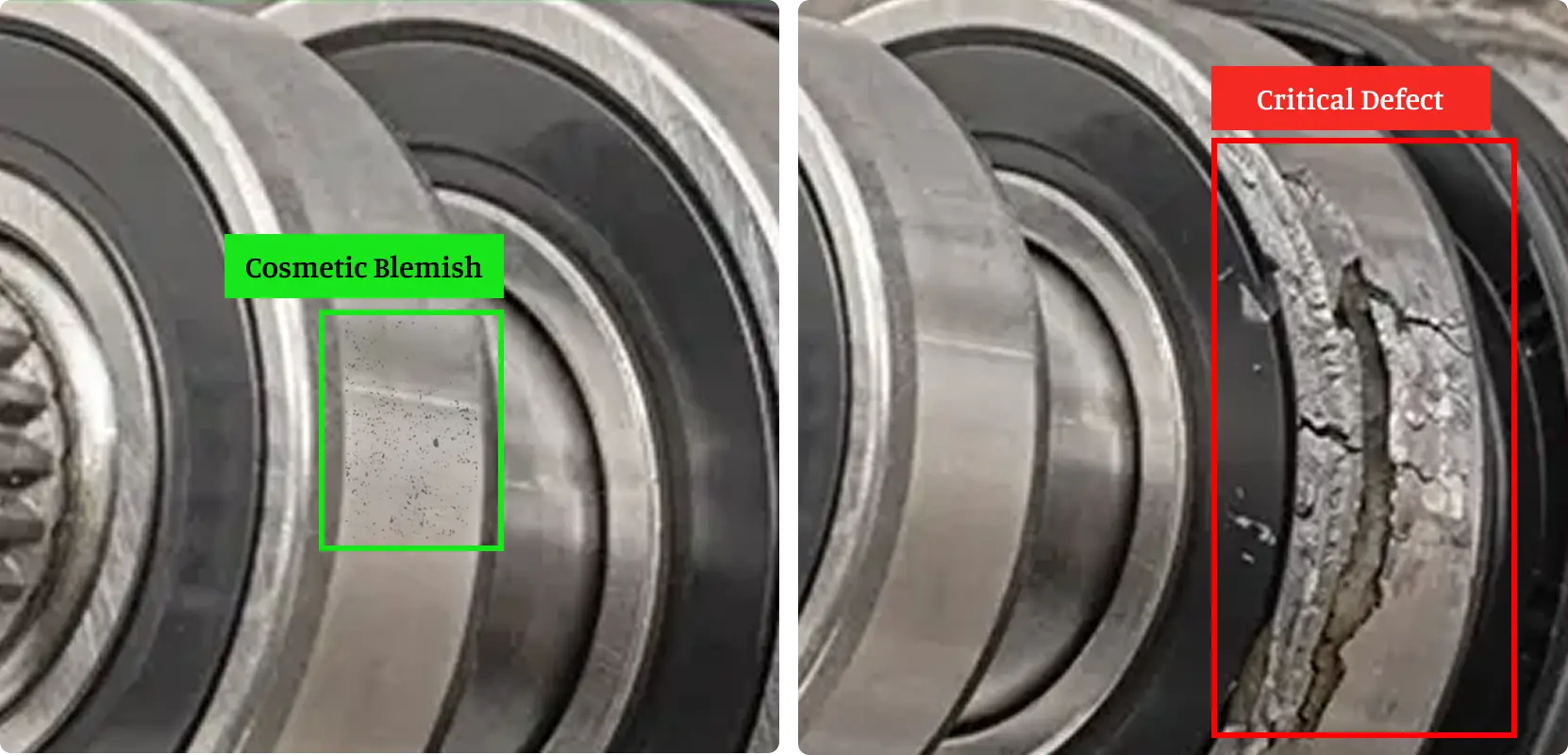
In modern manufacturing, not all defects are created equal – but they are often treated as if they are. A minor blemish on a surface finish might be completely inconsequential to a product’s function or safety, yet it could still trigger batch rejections, customer complaints, or costly rework. On the other hand, a seemingly trivial […]
Discover how transparent quality protocols reduce friction between OEMs and contract manufacturers, ensuring efficiency, trust, and better product outcomes.
In medical device manufacturing, quality isn’t just a benchmark — it’s a life-critical standard. Medical devices, particularly surgical and implantable devices, demand near-zero defect tolerance. Even the smallest flaw can trigger downstream consequences: from failed procedures and hospital brand-switching to FDA oversight that can last months. Yet, despite stringent quality control processes, batch rejections still […]